How Is Super Glue Made: Genius, Essential Guide
Super glue is made through a chemical process called anionic polymerization, primarily using a compound called cyanoacrylate. This reaction is triggered by moisture and quickly forms strong, durable bonds on various surfaces. It’s a fascinating blend of chemistry that results in the speedy adhesion we rely on for countless fixes.
Ever found yourself with a broken piece of your favorite mug, a loose chair leg, or a crafting project that needs a quick mend? Super glue is often our go-to solution, that little tube promising a strong, fast fix. But have you ever stopped to wonder, “How is super glue actually made?” It seems like magic, right? This amazing adhesive, also known as cyanoacrylate glue, has a surprisingly simple yet clever origin. Understanding its creation isn’t just for chemists; it’s a peek into the smart science behind everyday tools. Let’s dive down and uncover the genius behind this essential bonding agent. We’ll break down the process into easy-to-understand steps, so you’ll know exactly what makes this “super” stuff so effective.
The Secret Ingredient: Cyanoacrylate
At its heart, super glue is all about a special type of chemical called cyanoacrylate. Think of it as the main character in our gluing story. These molecules are incredibly reactive, meaning they love to join up with other molecules. This eagerness is precisely what makes them so good at sticking things together. The most common type of cyanoacrylate used in super glues is ethyl cyanoacrylate.
These cyanoacrylate monomers (that’s the scientific term for the individual building blocks) are kept stable in the bottle. They won’t just start sticking to everything in there. The real excitement begins when they come into contact with something that wakes them up and gets them working. And guess what that “something” usually is? It’s moisture!
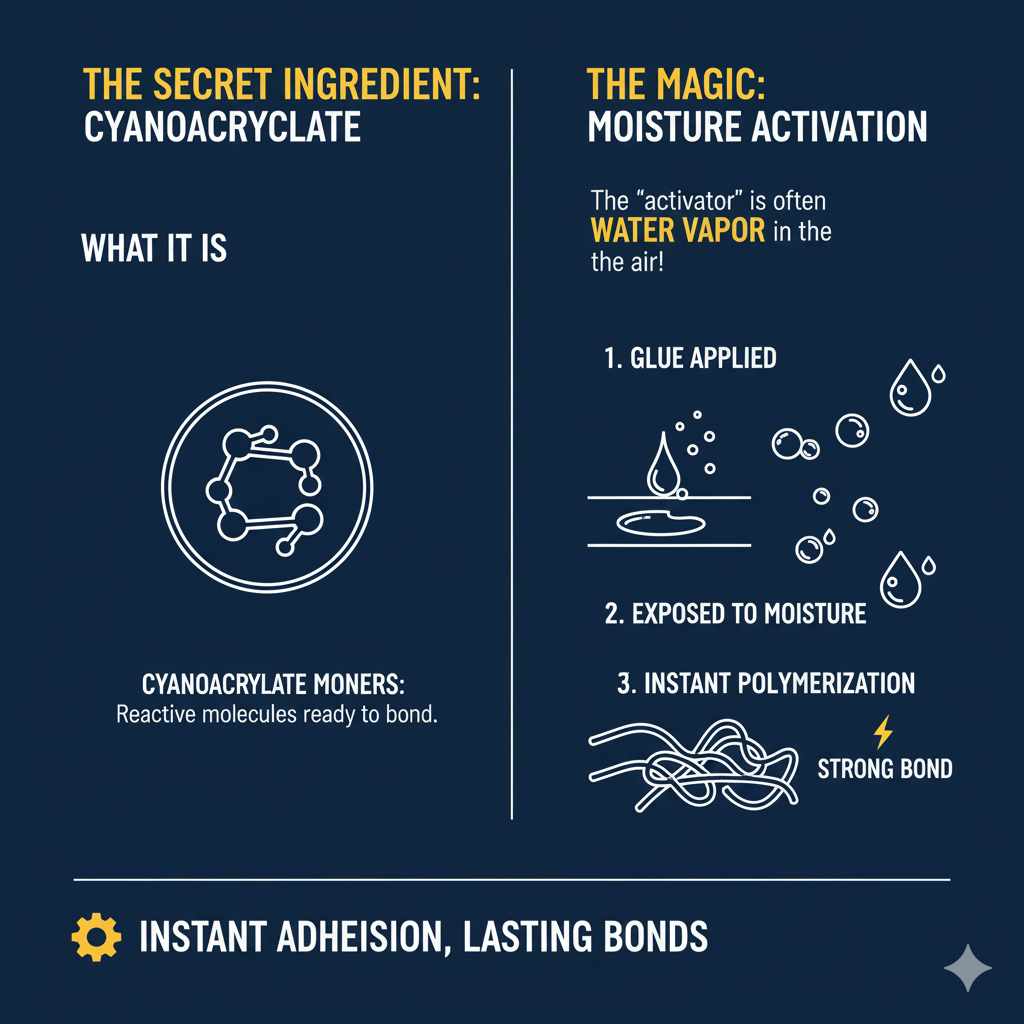
The Magic of Polymerization: How it All Sticks Together
The “super” in super glue comes from its ability to harden extremely quickly. This hardening process is called polymerization. When the cyanoacrylate monomers encounter even a tiny amount of moisture—like the water vapor present in the air or on the surfaces you’re trying to glue—a chemical reaction kicks off. This is known as anionic polymerization, an instant version of how plastics are often formed.
Here’s a simplified look at what happens:
- Initiation: A molecule from the moisture (like a hydroxide ion, OH-) attacks the cyanoacrylate monomer. This starts the chain reaction.
- Propagation: The activated monomer then attacks another monomer, and this process continues, forming long chains of molecules. Imagine tiny building blocks linking together, one after another, very, very quickly.
- Termination: Eventually, the chains stop growing. The result is a solid, hard plastic-like material that forms a strong bond between the surfaces it’s applied to.
This entire process happens in seconds, which is why super glue is so fast-acting. It grabs onto almost anything—wood, plastic, metal, ceramic, and even skin! Because it reacts with moisture, it’s best to store super glue in a dry place, and a little goes a long way. Too much moisture in the bottle and it can cure prematurely, turning into a useless solid lump.
The Manufacturing Process: From Lab to Your Toolbox
Creating super glue is a fascinating industrial process that involves carefully controlled chemical reactions. While the basic science is polymerization, the actual manufacturing involves several key stages to ensure purity, stability, and effectiveness. The primary raw material is formaldehyde, which is reacted with alkyl cyanoacetate. This creates a substance that is then heated to break down into cyanoacrylate monomers. These monomers are then purified. Special additives are often included to control the setting speed, viscosity (how thick or thin the glue is), and improve bond strength on specific materials. The final product is then bottled, often with a special nozzle to prevent clogging and premature curing.
Here’s a breakdown of the general steps involved in making super glue:
- Knoevenagel Condensation: The process usually starts with reacting formaldehyde (a simple organic compound) with an alkyl cyanoacetate (like methyl cyanoacetate or ethyl cyanoacetate). This forms a polymer, a larger molecule made up of repeating smaller units.
- Cracking (Depolymerization): This polymer is then heated under vacuum. This causes it to break down into its original monomer form, cyanoacrylate. This step requires precise temperature and pressure control to get pure monomers.
- Purification: The crude cyanoacrylate monomer is then purified, often through distillation, to remove any impurities or byproducts. This is crucial for the glue’s performance and shelf life.
- Stabilization: Pure cyanoacrylate monomers can be very unstable and react easily. To prevent premature curing in the bottle, stabilizers are added. These are typically acidic compounds that neutralize any basic substances that could initiate polymerization.
- Additives: Depending on the desired properties, other ingredients might be added. Thickeners can make the glue less runny, a common addition for woodworkers to control drips. Other additives can improve flexibility, impact resistance, or coloration.
- Packaging: Finally, the liquid is carefully bottled. Many containers have special caps or applicators designed to minimize air exposure and prevent the tip from becoming clogged, which is a common frustration for users.
Key Ingredients and Their Roles
Super glue isn’t just one single chemical; it’s a carefully formulated product. Understanding the role of each component helps appreciate its effectiveness:
| Component | Chemical Name/Type | Primary Role | Notes for Users |
|---|---|---|---|
| Monomer | Alkyl Cyanoacrylate (e.g., Ethyl Cyanoacrylate) | The core adhesive agent; forms long chains to create a bond. | This is what actually sticks things together when moisture is present. |
| Stabilizer | Acidic Compounds (e.g., Sulfur Dioxide, Phosphoric Acid) | Prevents premature polymerization in the bottle, ensuring shelf life. | Keeps the glue liquid and ready to use until you open it. |
| Thickener | Polymers (e.g., PMMA – Poly(methyl methacrylate)) or other agents | Controls viscosity, making the glue thicker and easier to apply on vertical surfaces or gaps. | This is why some super glues are runny like water, while others are more gel-like. |
| Accelerator (Optional) | Certain amines or other reactive compounds | Speeds up the curing process significantly, useful for porous materials or when a very fast bond is needed. | Can be helpful for quick repairs, but use sparingly as it can create a brittle bond. |
| Hardener/Curing Agent (Implicit) | Moisture (from air or substrates) | Initiates and drives the polymerization process. | This is why it’s important to have clean, dry surfaces for the best bond, but also why it works so fast. |
The primary ingredient, alkyl cyanoacrylate, is a marvel of chemistry. It’s a monomer that can rapidly polymerize, which is the process of linking many small molecules (monomers) into a long chain (polymer). This polymer forms the strong adhesive layer. The stabilizers are vital for shelf life; without them, the glue would harden in its tube. Thickeners are common in many super glues, especially those marketed for thicker applications or gap-filling, making them easier to control.
The Science Behind the Bond
When you apply super glue, you’re setting in motion a series of chemical reactions that create an incredibly strong bond. The key is the reaction between the cyanoacrylate monomers and the moisture present on the surfaces you’re joining, or even in the air. This moisture acts as an initiator for anionic polymerization.
Here’s a slightly more technical, though still simplified, look at the mechanism:
- Moisture as Initiator: The process begins with a weakly basic species, typically presented by trace moisture (forming hydroxide ions, O–H). This hydroxide ion attacks the electron-deficient carbon atom in the cyanoacrylate molecule.
- Chain Growth: This attack creates a new anion (a negatively charged molecule), which then reacts with another cyanoacrylate monomer. This continues, forming a long polymer chain. The process can be incredibly fast, occurring in fractions of a second.
- Polymer Formation: The resulting polymer, polycyanoacrylate, is a rigid plastic-like material. It forms a very strong, sometimes brittle, bond by penetrating microscopic pores and irregularities on the surfaces being joined.
This is why perfect surface preparation is key for the strongest bond. Clean, dry surfaces allow the monomers to react optimally. For very porous materials like unfinished wood, the glue can soak in, but it also means more moisture is available, leading to faster curing. Some industrial applications use specific activators or primers to ensure these reactions happen reliably and with extreme speed, even in challenging conditions. For comparison, traditional woodworking glues like PVA or epoxy rely on different mechanisms, often involving evaporation of water or a two-part chemical reaction that takes much longer.
Types of Super Glue and Their Differences
Not all super glues are created equal. While the core science is the same, manufacturers offer variations to suit different needs. Understanding these differences can help you choose the right one for your woodworking or DIY projects.
Cyanoacrylate Formulations
The most common distinction is based on the specific cyanoacrylate used:
- Ethyl-2-cyanoacrylate: This is the most widely used type. It’s a good all-rounder, offering a balance of strength, flexibility, and mild odor. It’s what you’ll find in most general-purpose super glues.
- Methyl-2-cyanoacrylate: This formulation tends to cure faster and create a stronger, but often more brittle, bond. It can also produce a more noticeable ‘blooming’ effect (a white powdery residue) around the bond line, which isn’t ideal for finished woodworking projects.
- Octyl-2-cyanoacrylate: This is a less common type for household use but is found in medical-grade adhesives. It cures much more slowly, produces less odor, and results in a more flexible bond, making it suitable for skin contact (like closing surgical wounds).
Viscosity Variations
Viscosity refers to how thick or runny the glue is. This is often adjusted with thickeners:
- Water-thin: These glues are very runny and can penetrate deep into porous materials or wick into tight cracks. They are excellent for quickly bonding small parts or reinforcing hairline fractures. However, they can also drip easily and are not ideal for filling gaps.
- Low Viscosity: Slightly thicker than water-thin, offering a bit more control without being overly viscous.
- Medium Viscosity: A good middle ground, offering more control than water-thin glues, allowing for some gap-filling and less dripping.
- Gel: These are the thickest types. They are designed to resist gravity, making them perfect for vertical surfaces or filling slightly larger gaps. Gel super glues are generally easier to control and apply precisely.
Specialty Formulations
Beyond the basic types, you’ll find super glues with added features:
- Flexible: Some formulations include rubber particles or other additives to create a more flexible bond that can withstand vibration or shock better. This is useful for materials that might flex or move.
- Impact-Resistant: Similar to flexible glues, these are engineered to absorb shock, reducing the chance of the bond failing under stress.
- Low Odor/Low Bloom: These are formulated to minimize the fumes and the white residue that can be a problem with faster-curing cyanoacrylates.
- Plastic Bonding: Specific formulations designed to work effectively on various types of plastics, which can sometimes be tricky to glue.
For woodworking, a medium viscosity or gel-type ethyl-2-cyanoacrylate is often preferred. They offer good control, can fill small gaps, and provide a fast, strong bond. You can find many great cyanoacrylate glues from reputable manufacturers like Gorilla Glue or Loctite that are suitable for a wide range of projects.
Safety First: Handling Super Glue
While super glue is incredibly useful, it demands respect. Its rapid bonding power means it can stick skin together instantly, and the fumes can be irritating. Always prioritize safety when working with it.
Tips for Safe Use:
- Ventilation: Always use super glue in a well-ventilated area. Open a window or work outdoors if possible. The fumes can be strong and irritating to the eyes and respiratory system. For extended use or in enclosed spaces, consider wearing a respirator mask approved for organic vapors.
- Eye Protection: Wear safety glasses or goggles. A tiny splash or mist can cause severe irritation or bonding of the eyelids.
- Skin Protection: Avoid direct skin contact. If you get super glue on your skin, don’t panic. It will eventually wear off as your skin naturally exfoliates. For faster removal, try soaking the affected area in warm, soapy water. You can also try gently rubbing with a pumice stone or using acetone-based nail polish remover (test on a small area first, as acetone can damage some finishes).
- Protect Surfaces: Cover your work surface with newspaper or a drop cloth to protect it from accidental drips. Super glue can bond to almost anything, and removing it from finished wood or countertops can be difficult.
- Storage: Store super glue in a cool, dry place, away from direct sunlight. Keep it out of reach of children and pets.
- Application: Apply only a small amount. Too much glue can weaken the bond and increase the chance of squeeze-out and unwanted sticking.
For beginners, starting with gel-type super glues can be a good way to minimize accidental drips and have more control during application. Understanding the safety precautions is just as important as knowing how to apply the glue itself. Remember, a little caution goes a long way in ensuring your projects are successful and safe.

Super Glue in Woodworking: Genius Applications
As a woodworker, I’ve come to rely on super glue for a variety of tasks where speed and a strong bond are essential. It’s not typically used for structural joints that will bear heavy loads (like those usually made with wood glue and joinery), but it excels in many other areas.
Common Woodworking Uses for Super Glue:
- Repairing Minor Chips and Dings: If you accidentally gouge a piece of wood or create a small ding during finishing, a tiny drop of super glue can fill the void. Once cured, it can be sanded flush and re-finished. For darker woods, you can sometimes fill the ding with wood dust collected during sanding, then apply super glue to bind it.
- Setting Small Parts Quickly: When attaching small decorative elements, inlays, or even temporarily holding small pieces in place for painting or finishing, super glue is a lifesaver. For example, if you’re adding a small wooden inlay pattern, a quick dab of super glue can secure it while a more permanent adhesive cures or while you work on other details.
- Reinforcing Veneer Edges: Sometimes, veneer edges can become brittle or slightly delaminated. A bit of water-thin super glue wicked into the edge can quickly re-bond it, preventing further peeling.
- Fixing Broken Tool Handles: If a small piece of a wooden tool handle breaks off, super glue can often provide a quick, effective repair.
- Temporary Clamping Assistance: While not a replacement for actual clamps, super glue can be used to tack two pieces together for a very short period, allowing you to get clamps properly positioned or finish a detail without the pieces shifting.
- Stabilizing Cracked Wood: For very fine cracks, particularly in decorative pieces or where strength isn’t paramount, super glue can be applied to prevent the crack from spreading.
It’s important to note that while super glue creates a strong bond, it’s often brittle. It doesn’t have the same flexibility or gap-filling capability as traditional wood glues like PVA or Titebond. It also doesn’t sand as easily as wood itself if you use too much. For more information on different types of wood glues and their applications, resources like those provided by the Woodworking Handbook can be very informative.
Frequently Asked Questions about Super Glue
What is super glue made of?
Super glue is primarily made of a chemical compound called cyanoacrylate. This is a monomer that can rapidly polymerize, forming a strong bond when exposed to moisture. Other ingredients include stabilizers to prevent premature curing and sometimes thickeners or accelerators.







