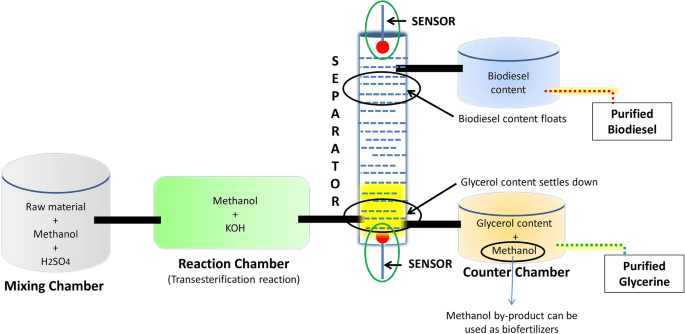
Will a Block of Ash Wood Float in Methanol? Discover Now
Have you ever wondered about the buoyancy of different materials? Imagine holding a block of ash wood in your hand.
It’s solid, sturdy, and naturally beautiful. But if you were to drop it in methanol, what would happen? Would it sink or float? This intriguing question not only piques your curiosity but also leads you into a world where science meets everyday observations.
By the end of this article, you’ll know whether ash wood will defy gravity or succumb to the depths of methanol. Get ready to dive into an exploration that challenges your assumptions and offers surprising insights.
Credit: www.mdpi.com
Properties Of Ash Wood
Ash wood is known for its medium density. It is strong and tough. The density of ash wood is around 0.6 to 0.8 grams per cubic centimeter. Methanol has a density of 0.79 grams per cubic centimeter. Ash wood’s density is close to methanol’s. This means ash wood might not float well in methanol. The composition of ash wood includes cellulose and lignin. These materials help make the wood strong. They also affect the wood’s weight and density.
Ash wood is used in making furniture. It is also used for flooring. People like it for its strength and beauty. It is also good for making sports equipment. Baseball bats and tool handles are often made from ash. Its toughness makes it a popular choice. The wood’s grain is straight, which is easy to work with. This makes it suitable for many uses.

Credit: pubs.rsc.org
Characteristics Of Methanol
Methanol is a simple alcohol. Its chemical formula is CH3OH. The structure has one carbon atom. It bonds with three hydrogen atoms. One more bond is with a hydroxyl group, OH. This makes it an alcohol. Methanol is a polar molecule. It mixes well with water. Its small size makes it quick to evaporate.
Methanol is a clear and colorless liquid. It has a slightly sweet smell. It is lighter than water. Methanol’s density is about 0.791 g/cm3. This makes it less dense than water. The boiling point is low at 64.7°C. The freezing point is -97.6°C. Methanol is highly flammable. It burns with a pale blue flame.
Buoyancy Principles
Archimedes’ Principle explains why objects float. It says that buoyancy is the force that keeps things afloat. If an object weighs less than the water it pushes away, it will float. This principle works in liquids like water and methanol. Ash wood is less dense than methanol, so it might float.
Many things affect buoyancy. Density matters a lot. If ash wood is less dense, it will float. The shape of the object also plays a role. Flat objects float better. The temperature of methanol can change buoyancy. Cold methanol might make things sink. Warm methanol can help things float. Always remember, buoyancy depends on many factors.

Credit: www.sciencedirect.com
Experiment Setup
You need a block of ash wood. Also, get some methanol. Use a clear container for the liquid. Make sure it can hold the block. Have a measuring scale ready. A ruler will help measure the block. Gather all these things before you start.
Fill the container with methanol. Make sure it’s not too full. Place the ash wood block in the container. Watch if it floats or sinks. Use the ruler to measure the height of the block. Record your observations. Check if the block touches the bottom. Note the wood’s position in the methanol.
Observations And Results
The ash wood block sat in methanol. It was easy to see. Methanol is clear. The block seemed to stay on the surface. This hints that it might be floating. No bubbles surrounded the block. The wood did not sink. Floating seemed likely.
We noted the block’s position. Time intervals were used. Every 5 minutes, a check was made. The block’s level stayed the same. This was recorded each time. The wood remained on top. All data was written down. The wood’s behavior was consistent.
Analysis Of Findings
Ash wood can float in water. Water is less dense than ash wood. Methanol is lighter than water. So, ash wood floats more easily in methanol. Methanol’s density is very low. This helps ash wood to float higher.
Density is like weight. If the wood is heavy, it sinks. If it is light, it floats. Ash wood is not too heavy. This helps it to float. Methanol’s low density makes floating easier. Light wood floats better in methanol. Denser wood may sink.
Implications And Applications
Ash wood is light. It is also strong. This makes it useful. People use it in sports. They make bats and paddles. It is also good for furniture. The wood looks nice. It is easy to shape. It is also durable. Ash wood is a favorite for builders. It is also used in tools. The wood is strong. It does not break easily. This makes it a good choice. People also use it in flooring. The wood is tough. It can handle a lot of use.
Ash trees grow fast. This makes them a good resource. People can plant more trees. They can grow quickly. This helps the environment. Using ash wood is sustainable. It does not harm forests. People need to manage forests well. This keeps them healthy. The wood is biodegradable. It breaks down naturally. This is good for the planet. Methanol is a chemical. It can harm nature. Careful use is important. This protects the earth.
Frequently Asked Questions
Will A Block Of Ashwood Float In Methanol?
Yes, a block of ashwood will float in methanol. Ashwood’s density is lower than methanol’s density. Methanol’s density is approximately 0. 791 g/cm³, while ashwood’s density is roughly 0. 67 g/cm³. This density difference ensures buoyancy, allowing ashwood to float easily in methanol.
Does Wood Ash Float?
Wood ash typically doesn’t float in water. Its particles are denser than water, causing them to sink. However, very fine particles might momentarily stay suspended before settling.
Will Any Substance Float In Methanol Why?
Yes, substances can float in methanol if their density is lower than methanol’s density, which is 0. 791 g/cm³. Objects with densities higher than methanol will sink. Floating depends on the object’s material and density relative to methanol.
Does Wood Contain Methanol?
Wood naturally contains small amounts of methanol. Methanol is released during the wood’s breakdown or burning process. It’s a common byproduct in wood-based activities like woodworking or pyrolysis. Proper ventilation reduces risks associated with methanol exposure. Always handle wood with care to ensure safety.
Conclusion
Ash wood floats in methanol due to its low density. Methanol’s density is higher than ash wood, allowing it to float. This simple experiment shows density differences in materials. Whether for fun or learning, it’s clear. Understanding these principles can help in various fields.
Simple science offers valuable insights into everyday phenomena. Explore more materials and their interaction with liquids. Discover the fascinating world of density and buoyancy. Learning through experimentation enhances knowledge. Ash wood and methanol provide a clear example. An enjoyable way to grasp scientific concepts.